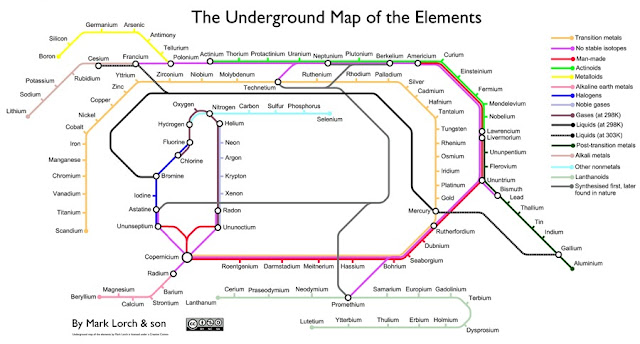
Elements - (Poorly) Visualized as a Tube Map
Mark Lorch, at the Guardian, redoes the periodic table of elements as a tube map. As he puts it
Each line on my map contains elements that share a characteristic. Sometimes these correspond directly to regions of the periodic table, so the alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids and non-metals, halogens, noble gases, actinoids and lanthanoids are all there (see the jargon buster below).
As well as these there are characteristics that aren't immediately apparent from periodic tables, for example liquids (at room temperature and slightly above) have their own line, and there are lines for gases and elements with no stable isotopes (ie they are radioactive).
I also threw in a smattering of history: elements that are manmade get a line of their own, as do those that were first made in a lab before being discovered in nature.The result is a disaster - you can't really tell anything from this. Compare this w/ the periodic table of elements below, which is clear, concise, and brilliant. In Mark Lorch's words
The periodic table is an awesome piece of information organisation. By following one cardinal rule – all elements appear in order of their atomic number – and then carefully restricting the number of elements in each row (or period), clear patterns emerge.
For example, the first column (or group) contains the alkali metals. These are all soft, shiny and highly reactive (remember chemistrylessons where the teacher dropped sodium or even potassium into water). The opposite end of the table couldn't be more different. It contains the noble gases, which are all inert and unreactive
But the really amazing thing about the table, created in 1869 by Dmitri Mendeleev, is that, without fail, it is totally inclusive. Every element ever discovered or synthesised has its place setting at the table. So when Mendeleev compiled the first draft he was able to predict the existence of, then unknown, elements by the obvious gaps in the table. That is why all the periodic table imitations will always be vastly inferior (with the possible exception of the periodic table of irrational nonsense).



Comments